(OBP-301)
(OBP-301)
- Outline / Target indication / Current status
- Preclinical studies and proof of concept
- Clinical Development Strategy and Program
The lifecycle commercialization program of Telomelysin employs a considered expansion strategy
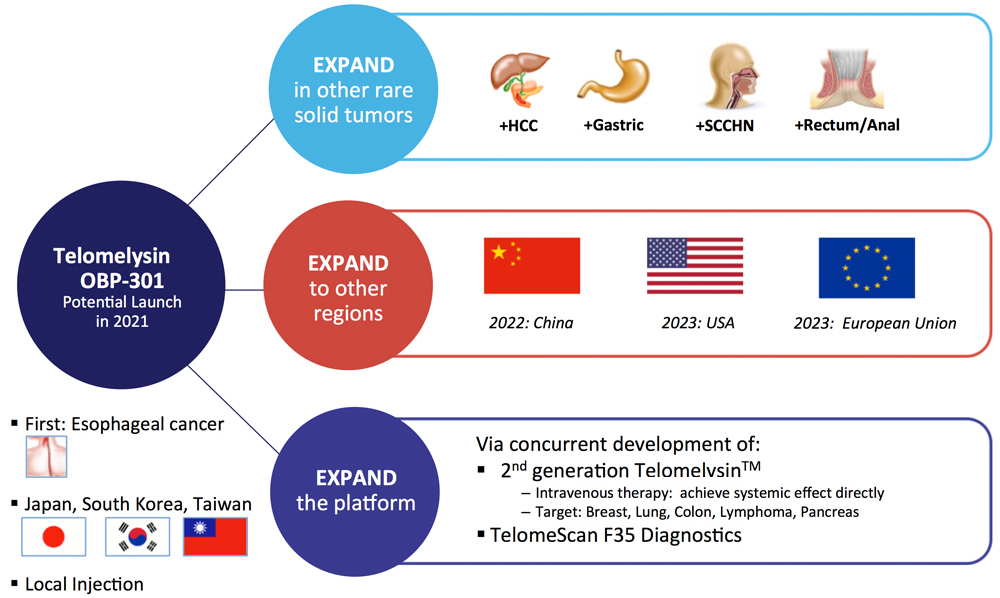
Potential 2021 launch in esophageal cancer given significant unmet needs
High Mortality
Rates
Rates
90%
90% of patients diagnosed with esophageal cancer will die within 2 yearsLimited
improvements
improvements
Non-operative Settings
Advanced disease at presentation is managed with non-operative approaches (SOC: cisplatin, 5-FU, and radiotherapy alone) which have not seen improvement in outcome for 25 yearsLimited
Options
Options
Metastatic Setting
Metastatic esophageal cancer and GEJ cancer have limited approved treatment options and modest efficacy (Nivo and pembro, range of 10-15% in the 3L setting)Patient
Population
Population
High Potential
Pembrolizumab-refractory: With pembrolizumab’s recent approval in third-line setting, there will be many eligible patients refractory to pembrolizumab that will need another option
Older population: Low awareness in elderly allows a huge potential to demonstrate improved outcomes in the older populationFDA
Designations
Designations
Opportunity
To pursue Orphan Drug Designation and Breakthrough Therapy Designation
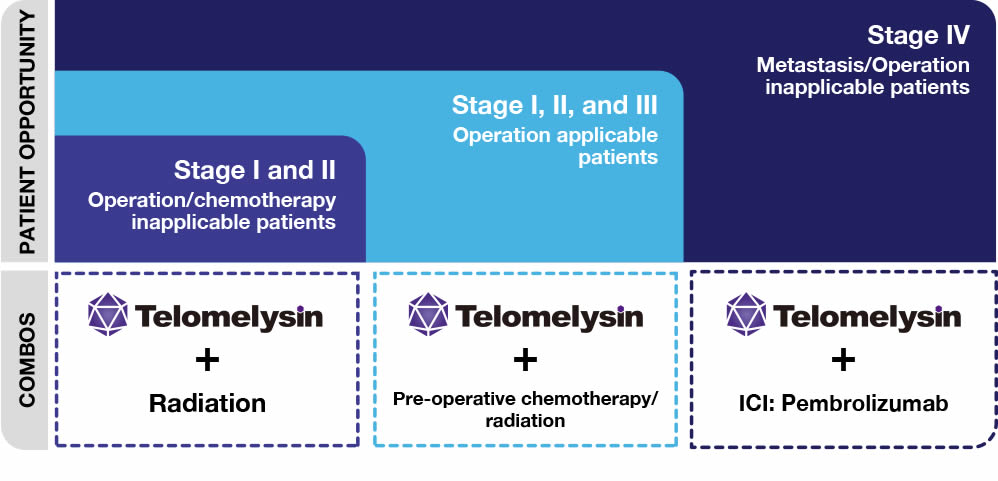
Given the unmet need in esophageal cancer, Telomelysin has opportunities across different patient types - and in combo with different modalities
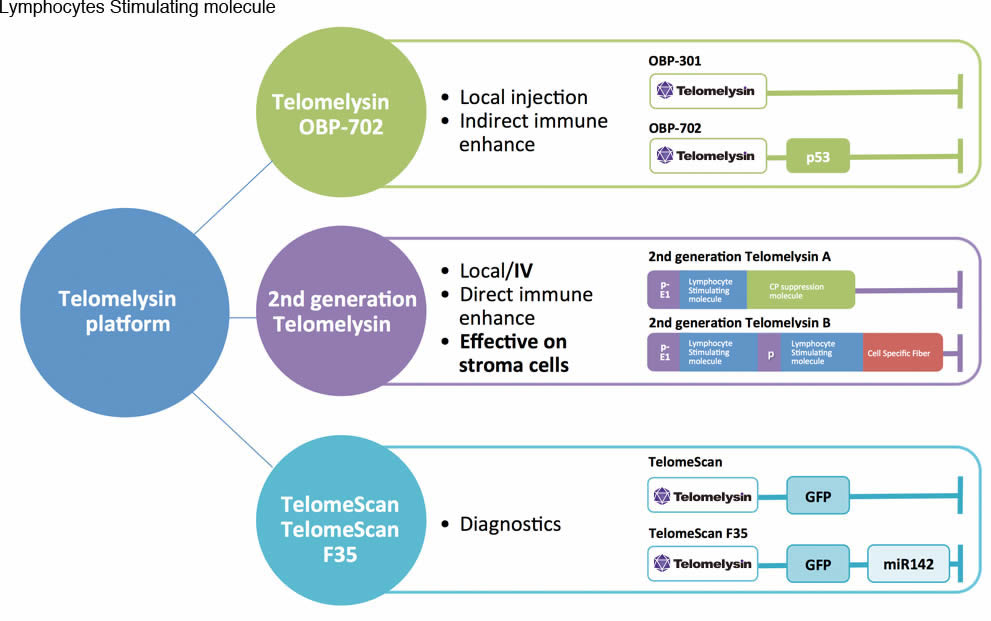
Telomelysin Platform