(OBP-301)
(OBP-301)
- Outline / Target indication / Current status
- Preclinical studies and proof of concept
- Clinical Development Strategy and Program
Outline
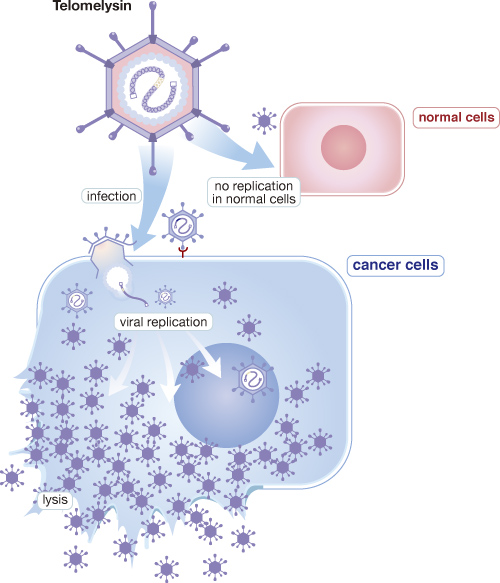
Telomelysin (OBP-301) is a gene-modified oncolytic adenovirus in which selectively replicate in cancer cells by introducing human telomerase reverse transcriptase (hTERT) promotor. Oncolytic adenovirus has much potential for cancer immunotherapy because its viral replication is highly immunogenic, and oncolysis induced by such virus releases tumor antigen and provides costimulatory danger signals. From the result of phase 1 clinical study in the US, Telomelysin showed abscopal effect, which non-injected tumor as well as injected tumor was regressed in melanoma patients after single injection into one single tumor and found that not only increasing infiltration of CD8 and antigen presenting cells but diminishing Treg cells in injected tumor site.
In preclinical studies for Telomelysin, Oncolys has demonstrated effective anti-tumor activity on various cancer cells, and there was no finding that may bring safety concerns in toxicological studies as well as bio-distribution study. Oncolys completed the phase 1 clinical trial for solid tumors in the US with 16 subjects for single dosing and 6 subjects for repeated dosing. Most of the adverse events observed in the study was mild to moderate and transient, and there was a sign of efficacy showing tumor shrinkage in 8 out of 12 evaluable patients.
In Japan, investigator initiated clinical research for esophageal cancer in combination with radiotherapy was conducted in Okayama University. The research, completed in 2018, resulted that 8 out of 13 patients showed CR (complete response), and CD8 T cell infiltration was observed in tumor area. Based on such positive data, Oncolys started a phase 1 study in 2017, which is has completed on September 2019 and a Japanese pharmaceutical company Chugai Pharmaceutical is conducting phase 2 study, based on the exclusive license agreement for Telomelysin concluded between Chugai and Oncolys on 8 April, 2019.
Target indication
Gastrointestinal cancers (esophageal cancer, gastric cancer, etc.), head and neck cancer, and hepatocellular carcinoma
Current status
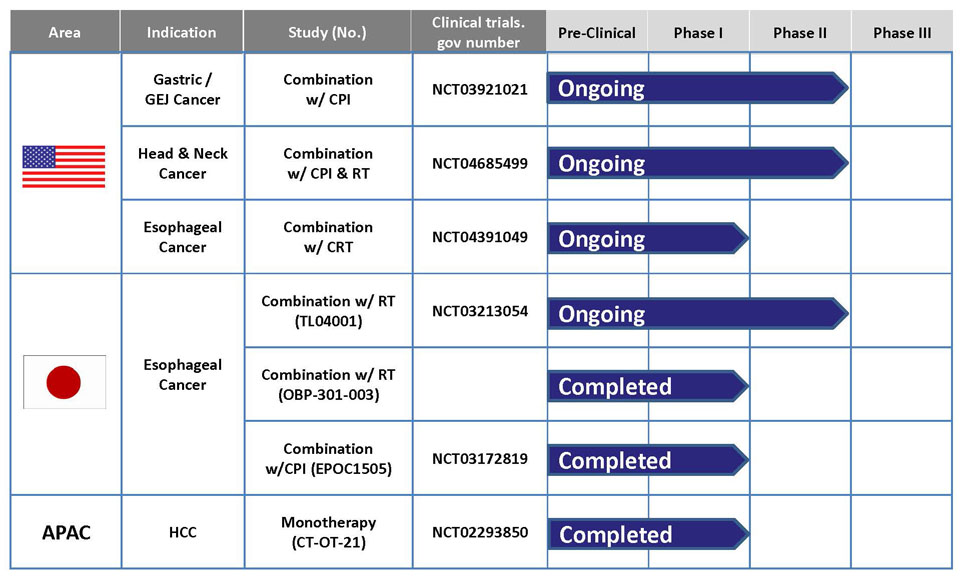
- For progress of development, refer to "Outline of Our Pipeline: Progress chart of pipeline.
- For details, refer to "Research and Development Activities" in the latest financial statements.
(Countries where patent was issued)
Japan, United States, Europe, South Africa, Singapore, New Zealand, Australia, China, Hong Kong, South Korea, Canada.
Telomelysin & Current Development Status